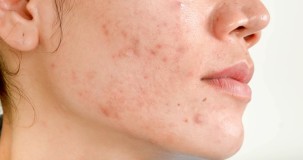
 Symptoms
Symptoms
 Pregnancy
Pregnancy
 Pregnancy
Pregnancy
 Pregnancy
Pregnancy
 Baby
Baby
 Baby
Baby
 Pregnancy
Pregnancy
 Benefits
Benefits
 Benefits
Benefits
Are prions alive? Discover the truth: are prions alive? Understand the science behind prions with this insightful blog post. Explore their intriguing characteristics and find out whether they fall into the realm of living organisms.
What are Prions?
Prions are misfolded proteins that can induce the misfolding of normal proteins they come into contact with. These misfolded proteins have a higher propensity to aggregate, forming clumps that disrupt cellular activity and lead to tissue damage. Prions are primarily associated with transmissible spongiform encephalopathies (TSEs), a group of diseases affecting the brain and central nervous system.
Unique Properties of Prions
One of the most distinctive features of prions is their ability to convert normal proteins into the misfolded prion form. This conversion occurs through a template-based mechanism, where the misfolded prion acts as a template to induce the misfolding of normal proteins. This self-propagating nature is what distinguishes prions from other protein aggregates involved in neurodegenerative disorders.
Furthermore, prions are incredibly resistant to traditional methods of inactivation, such as heat, radiation, or chemical agents. They can withstand extreme conditions and remain infectious for long periods, posing a challenge for sterilization procedures and disease prevention strategies.
The Prion Diseases
The most well-known prion disease in humans is Creutzfeldt-Jakob disease (CJD), which manifests as a rapidly progressing dementia. Other prion diseases include variant CJD, Gerstmann-Sträussler-Scheinker syndrome, and fatal familial insomnia.
In animals, prion diseases have been observed in various species, including bovine spongiform encephalopathy (BSE or "mad cow disease") in cattle and chronic wasting disease in deer and elk. These diseases have significant economic and ecological implications due to their transmissible nature and potential to spread across populations.
Prions and the Controversy of Life
The classification of prions as non-living particles challenges our conventional understanding of life. Unlike viruses, prions lack nucleic acids, the genetic material essential for replication. They are not composed of cells or possess any metabolic processes.
However, prions can hijack the host's cellular machinery and replicate themselves by converting normal proteins into the misfolded prion form. This unique ability blurs the line between living and non-living entities and has spurred intense debates among scientists regarding the definition of life.
Research and Implications
Understanding the mechanisms behind prion diseases is vital for developing effective treatments and preventive measures. Researchers are studying the structural properties of misfolded prions and investigating potential therapeutic targets to halt their propagation.
The discovery of prions has also raised concerns in various sectors, such as public health, agriculture, and food safety. Strict regulations and monitoring systems have been implemented to prevent the spread of prion diseases in both human and animal populations.
In conclusion
Prions occupy a unique and perplexing space within the realm of infectious agents. While they lack the traditional characteristics of living organisms, their ability to propagate and cause devastating diseases cannot be ignored. Continued research into prions will undoubtedly shed further light on the nature of life and may uncover potential breakthroughs in the field of neurodegenerative diseases.
No, prions are not considered to be living organisms. They do not have a cellular structure and lack the essential properties of life, such as metabolism and reproduction.
2. What are prions made of?Prions are made up of misfolded proteins. These misfolded proteins can induce other proteins with the same amino acid sequence to also misfold, leading to the formation of aggregates and causing disease.
3. Can prions be destroyed or inactivated?Prions are highly resistant to many common methods of destruction or inactivation, such as heat, radiation, chemicals, and disinfectants. They can withstand extreme conditions and retain their infectivity for long periods of time.
4. How do prions cause diseases?Prions cause diseases by converting normal, soluble proteins into abnormal, insoluble forms. These abnormal proteins then accumulate in the brain tissue, causing neuronal damage and leading to the development of neurodegenerative diseases like Creutzfeldt-Jakob disease.
5. Can prion diseases be transmitted between species?Yes, prion diseases can be transmitted between species. Certain prion diseases, such as bovine spongiform encephalopathy (BSE) in cows, can be transmitted to humans through the consumption of contaminated meat products. This transmission is thought to occur due to the ability of prions to cross species barriers and induce misfolding of related proteins.
 LATEST ARTICLES
LATEST ARTICLES

At what stage in pregnancy does heartburn start?

Can a pregnancy test be wrong?

Can constipation hurt the baby during pregnancy?

Can a pregnancy test be wrong if taken too early?

Can a pregnancy test read wrong?

Are meds safe during pregnancy?

Can a UTI cause a false positive pregnancy test?

Can breasts be sore without pregnancy?

Can 2 faint positive pregnancy tests be wrong?

Can a pregnancy test change to positive after 10 mins?

Can a pregnancy test be positive one day and negative the next?

Can corpus luteum cyst cause positive pregnancy test?

Can a pregnancy be successful with low hCG?

Are pregnancy tests accurate at night?

Can a pregnancy test lie about being positive?

Are pregnancy bumps hard or soft?

Can clearblue detect 1 week pregnancy?

Are you dry in early pregnancy?

Can bananas cause heartburn during pregnancy?

Can autism be detected during pregnancy?

Can a positive pregnancy test can be wrong?

Can a pregnancy test be positive at 1 week?

Can AFE happen during pregnancy?
 POPULAR ARTICLES
POPULAR ARTICLES

Am I bloated or just fat?
Am I bloated or do I have an ovarian cyst?

Am I bloated or fat?

Are blackouts a symptom of depression?
Are blisters symptoms of diabetes?
Are blackouts a symptom of anxiety?

Are apples good for pregnancy?

Are any medications Pregnancy Category A?

Are bananas good for pregnancy?

Are baby kicks stronger at 20 weeks?